Wilton Industries, Inc. of Naperville, Illinois is initiating a voluntary national recall of select lots of Rainbow Chip Crunch Sprinkles And Rainbow Sprinkles Mix due to undeclared milk. Although the product label does indicate that it may contain milk, this recall is being conducted because some samples of the product were found to contain milk.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/wilton-brands-issues-allergy-alert-possible-presence-undeclared-milk-two-sprinkle-products
Wilton Brands Issues Allergy Alert on Possible Presence of Undeclared Milk in Two Sprinkle Products
Summary
Company Announcement Date: June 02, 2022
FDA Publish Date: June 06, 2022
Product Type: Food & Beverages
Reason for Announcement: Undeclared Milk
Company Name: Wilton Industries, Inc.
Brand Name: Wilton
Product Description: Rainbow Chip Crunch Sprinkles and Rainbow Sprinkles Mix
Thursday, June 9, 2022
CA Company Recalls Strawberry Tea Products After Produced with Strawberries Linked to Hepatitis A Outbreak
Urban Remedy, a California based producer of fresh organic, ready-to-eat meals, juices and snacks, is voluntarily recalling Urban Remedy Organic Revitalizing Tea Tonic Strawberry Hibiscus Rose (LOT 1232 BEST BY 7/17/2022) because it has the potential to be contaminated with Hepatitis A. Urban Remedy contracts Youngstown Grape Distributors Inc. to co-manufacture this product. The product may contain fresh organic strawberries linked to the FDA outbreak investigation of FreshKampo organic strawberries.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/urban-remedy-recalls-urban-remedy-organic-revitalizing-tea-tonic-strawberry-hibiscus-rose-due
Urban Remedy Recalls Urban Remedy Organic Revitalizing Tea Tonic - Strawberry Hibiscus Rose Due to Possible Hepatitis A Contamination
Summary
Company Announcement Date: June 03, 2022
FDA Publish Date: June 05, 2022
Product Type: Food & Beverages
Reason for Announcement: Potential to be contaminated with Hepatitis A.
Company Name: Urban Remedy
Brand Name: Urban Remedy
Product Description: Organic Revitalizing Tea Tonic Strawberry Hibiscus Rose
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/urban-remedy-recalls-urban-remedy-organic-revitalizing-tea-tonic-strawberry-hibiscus-rose-due
Urban Remedy Recalls Urban Remedy Organic Revitalizing Tea Tonic - Strawberry Hibiscus Rose Due to Possible Hepatitis A Contamination
Summary
Company Announcement Date: June 03, 2022
FDA Publish Date: June 05, 2022
Product Type: Food & Beverages
Reason for Announcement: Potential to be contaminated with Hepatitis A.
Company Name: Urban Remedy
Brand Name: Urban Remedy
Product Description: Organic Revitalizing Tea Tonic Strawberry Hibiscus Rose
Alabama Seafood Processor Recalls Crabmeat After FDA Finds Listeria in Cooking Operations
Irvington Seafood of Irvington, AL, is recalling its 1 lb. packages of "Crabmeat: Jumbo, Lump, Finger, and Claw meat" because they have the potential to be contaminated with Listeria monocytogenes
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/irvington-seafood-recalls-crabmeat-jumbo-lump-finger-and-claw-meat-because-possible-health-risk
Irvington Seafood Recalls “Crabmeat: Jumbo, Lump, Finger, and Claw Meat” Because of Possible Health Risk
Summary
Company Announcement Date: June 02, 2022
FDA Publish Date: June 03, 2022
Product Type: Food & Beverages
Reason for Announcement: Potential to be contaminated with Listeria monocytogenes
Company Name: Irvington Seafood Inc.
Brand Name: Irvington Seafood
Product Description: Crabmeat: Jumbo, Lump, Finger, and Claw meat
No illnesses have been reported to date in connection with this problem.
The potential for contamination was noted on 05/27/22 after FDA tested on 05/09/22. FDA revealed the presence of Listeria monocytogenes on cooking equipment and cooking room. No crabmeat has been tested but product may have potentially been contaminated with Listeria monocytogenes due to cross contamination.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/irvington-seafood-recalls-crabmeat-jumbo-lump-finger-and-claw-meat-because-possible-health-risk
Irvington Seafood Recalls “Crabmeat: Jumbo, Lump, Finger, and Claw Meat” Because of Possible Health Risk
Summary
Company Announcement Date: June 02, 2022
FDA Publish Date: June 03, 2022
Product Type: Food & Beverages
Reason for Announcement: Potential to be contaminated with Listeria monocytogenes
Company Name: Irvington Seafood Inc.
Brand Name: Irvington Seafood
Product Description: Crabmeat: Jumbo, Lump, Finger, and Claw meat
NJ Bakery Recalls Carrot Cake Squares Due to Undeclared Walnuts
Leonard Novelty Bakery of Moonachie NJ announced today a recall involving Carrot Cake Squares sold in the store's Bakery departments due to undeclared walnuts
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/leonard-novelty-bakery-announced-recall-involving-carrot-cake-squares-due-undeclared-walnuts
Leonard Novelty Bakery Announced a Recall Involving Carrot Cake Squares Due to Undeclared Walnuts
Summary
Company Announcement Date: May 17, 2022
FDA Publish Date: June 02, 2022
Product Type: Food & Beverages
Reason for Announcement: Undeclared walnuts
Company Name: Leonard Novelty Bakery
Brand Name: Leonard Novelty Bakery
Product Description: Carrot cake squares
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/leonard-novelty-bakery-announced-recall-involving-carrot-cake-squares-due-undeclared-walnuts
Leonard Novelty Bakery Announced a Recall Involving Carrot Cake Squares Due to Undeclared Walnuts
Summary
Company Announcement Date: May 17, 2022
FDA Publish Date: June 02, 2022
Product Type: Food & Beverages
Reason for Announcement: Undeclared walnuts
Company Name: Leonard Novelty Bakery
Brand Name: Leonard Novelty Bakery
Product Description: Carrot cake squares
Missouri Cheese Manufacturer Recalls Cheese Due to Listeria
Paris Brothers, Inc., of Kansas City, Missouri is recalling several specific cheese products listed below because they have the potential to be contaminated with Listeria monocytogenes. This recall is the result of routine sampling by the FDA, which revealed the presence of Listeria monocytogenes.
The cheeses were produced on May 4, 5, and 6, 2022 are the only products in the recall.
No illnesses have been reported to date.
The cheeses were produced on May 4, 5, and 6, 2022 are the only products in the recall.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/paris-brothers-inc-voluntary-limited-recall
Paris Brothers Inc. Voluntary Limited Recall
Summary
Company Announcement Date: June 01, 2022
FDA Publish Date: June 01, 2022
Product Type: Food & Beverages Cheese/Cheese Product
Reason for Announcement: Potential to be contaminated with Listeria monocytogenes
Company Name: Paris Brothers, Inc.
Brand Name: Paris Brothers, Cottonwood River, D’amir, more
Product Description: Cheese products
Paris Brothers Inc. Voluntary Limited Recall
Summary
Company Announcement Date: June 01, 2022
FDA Publish Date: June 01, 2022
Product Type: Food & Beverages Cheese/Cheese Product
Reason for Announcement: Potential to be contaminated with Listeria monocytogenes
Company Name: Paris Brothers, Inc.
Brand Name: Paris Brothers, Cottonwood River, D’amir, more
Product Description: Cheese products
Tuesday, May 31, 2022
Salad Kit Recalled After Consumer Finds Wrong Salad Dressing
Taylor Farms Retail of Guadalupe, California is voluntarily recalling a single production day of Trader Joe’s Lemony Arugula Basil Salad Kit due to the potential of undeclared wheat and eggs contained in the kit. The product may contain wheat and eggs that are not declared on the label. The issue was identified when a consumer reported an incorrect dressing packet within the kit.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/california-firm-issues-voluntary-product-recall-due-undeclared-wheat-and-egg-trader-joes-lemony
California Firm Issues Voluntary Product Recall Due to Undeclared Wheat and Egg in Trader Joe’s Lemony Arugula Basil Salad Kit
Summary
Company Announcement Date: May 27, 2022
FDA Publish Date: May 28, 2022
Product Type: Food & Beverages
Reason for Announcement: Undeclared wheat and eggs
Company Name: Taylor Farms Retail
Brand Name: Trader Joe’s
Product Description: Lemony Arugula Basil Salad Kit
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/california-firm-issues-voluntary-product-recall-due-undeclared-wheat-and-egg-trader-joes-lemony
California Firm Issues Voluntary Product Recall Due to Undeclared Wheat and Egg in Trader Joe’s Lemony Arugula Basil Salad Kit
Summary
Company Announcement Date: May 27, 2022
FDA Publish Date: May 28, 2022
Product Type: Food & Beverages
Reason for Announcement: Undeclared wheat and eggs
Company Name: Taylor Farms Retail
Brand Name: Trader Joe’s
Product Description: Lemony Arugula Basil Salad Kit
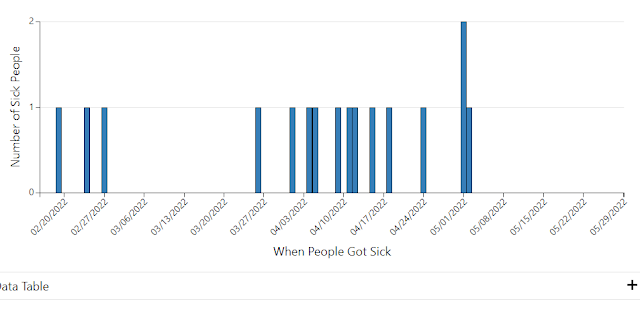
FDA Issues Health Alert Regarding Hepatitis A Linked to Fresh Strawberries
FDA is investigating an outbreak of Hepatitis A virus that has been linked to strawberries that was sold at various retail store chains. The product has already exceeded its shelf-life, so there are not likely to be any fresh product, but the concern would be if someone froze the strawberries.
To this point, there have been 17 cases with 12 hospitalizations. Illness onset dates range from March 28 – April 30, 2022.
The product was sold as FreshKampo or HEB brand organic strawberries purchased between March 5, 2022, and April 25, 2022.
https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-hepatitis-virus-strawberries-may-2022
Outbreak Investigation of Hepatitis A Virus: Strawberries (May 2022)
To this point, there have been 17 cases with 12 hospitalizations. Illness onset dates range from March 28 – April 30, 2022.
The product was sold as FreshKampo or HEB brand organic strawberries purchased between March 5, 2022, and April 25, 2022.
https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-hepatitis-virus-strawberries-may-2022
Outbreak Investigation of Hepatitis A Virus: Strawberries (May 2022)
Case Counts
Total U.S. Illnesses: 17
Hospitalizations: 12
Deaths: 0
Last illness onset: April 30, 2022
States with Cases: CA (15), MN (1), ND (1)
Total U.S. Illnesses: 17
Hospitalizations: 12
Deaths: 0
Last illness onset: April 30, 2022
States with Cases: CA (15), MN (1), ND (1)
Friday, May 27, 2022
CDC and FDA Provide Update on Peanut Butter Recall (5/26/22)
CDC and FDA provided an update to the JIF peanut butter recall due to Salmonella related cases. There are now 16 reported illnesses with 2 hospitalizations in 12 different states. The recall was initially announced on May 21 with 14 reported cases. From the CDC, "Since the last update on May 21, 2022, two more illnesses have been reported. As of May 25, 2022, a total of 16 people infected with the outbreak strain of Salmonella Senftenberg have been reported from 12 states (see map). Illnesses started on dates ranging from February 19, 2022, through May 2, 2022 (see timeline)."
Multiple companies have issued recalls after using the recalled peanut butter as part of their product.
Salmonella Outbreak Linked to Peanut Butter | CDC
Salmonella Outbreak Linked to Peanut Butter
Posted May 26, 2022
Fast Facts
Illnesses: 16
Hospitalizations: 2
Deaths: 0
States: 12
Recall: Yes
Investigation status: Active
Multiple companies have issued recalls after using the recalled peanut butter as part of their product.
Salmonella Outbreak Linked to Peanut Butter | CDC
Salmonella Outbreak Linked to Peanut Butter
Posted May 26, 2022
Fast Facts
Illnesses: 16
Hospitalizations: 2
Deaths: 0
States: 12
Recall: Yes
Investigation status: Active
FDA Issues Warning Letter to NC Sprout Growing Facility
FDA issued a Warning Letter to a sprout growing company for issues involving pathogen control as well as making unsubstantiated medical claims regarding the benefits of sprouts.
The company did not discontinue the use of all seeds from a lot which were known to had reason to believe may have been contaminated with a pathogen, and did not ensure that sprouts grown from that lot of seeds did not enter commerce as required by 21 CFR 112.142(b)(1). Specifically, when spent sprout irrigation water (SSIW) samples corresponding to specific seed lots tested positive for pathogens, the company did not discontinue the use of seeds from those lots.
The company did not clean and sanitize food contact surfaces used to grow, harvest, pack, or hold sprouts before contact with seeds or beans used to grow sprouts, as required by 21 CFR 112.143(b)). for example, after treatment the bags are rinsed and reused with no additional cleaning or sanitization performed prior to further contact with seeds or beans.
The environmental monitoring plan did not specify sample collection sites including appropriate food contact and non-food contact surfaces of equipment, and other surfaces within the growing, harvesting, packing, and holding environment, as required by 21 CFR 112.145(c)(3). The HACCP Manager stated that swab locations are determined at random, and the time of sampling varies and may include times when production is not occurring.
FDA reviewed the website listed for Broccoli Sprouts, Deli Blend, and Salad Blend product labels at the internet address, www.sunnycreekfarm.com, in November 2021. The claims on the website establish that the products are drugs under section 201(g)(1)(B) of the Act [21 U.S.C. 321(g)(1)(B)] because they are intended for use in the cure, mitigation, treatment, or prevention of disease. As explained further below, introducing or delivering these products for introduction, into interstate commerce for such uses violates the Act.
https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/sunny-creek-farm-inc-617664-02022022
Sunny Creek Farm, Inc.
MARCS-CMS 617664 — FEBRUARY 02, 2022
The company did not discontinue the use of all seeds from a lot which were known to had reason to believe may have been contaminated with a pathogen, and did not ensure that sprouts grown from that lot of seeds did not enter commerce as required by 21 CFR 112.142(b)(1). Specifically, when spent sprout irrigation water (SSIW) samples corresponding to specific seed lots tested positive for pathogens, the company did not discontinue the use of seeds from those lots.
The company did not clean and sanitize food contact surfaces used to grow, harvest, pack, or hold sprouts before contact with seeds or beans used to grow sprouts, as required by 21 CFR 112.143(b)). for example, after treatment the bags are rinsed and reused with no additional cleaning or sanitization performed prior to further contact with seeds or beans.
The environmental monitoring plan did not specify sample collection sites including appropriate food contact and non-food contact surfaces of equipment, and other surfaces within the growing, harvesting, packing, and holding environment, as required by 21 CFR 112.145(c)(3). The HACCP Manager stated that swab locations are determined at random, and the time of sampling varies and may include times when production is not occurring.
FDA reviewed the website listed for Broccoli Sprouts, Deli Blend, and Salad Blend product labels at the internet address, www.sunnycreekfarm.com, in November 2021. The claims on the website establish that the products are drugs under section 201(g)(1)(B) of the Act [21 U.S.C. 321(g)(1)(B)] because they are intended for use in the cure, mitigation, treatment, or prevention of disease. As explained further below, introducing or delivering these products for introduction, into interstate commerce for such uses violates the Act.
https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/sunny-creek-farm-inc-617664-02022022
Sunny Creek Farm, Inc.
MARCS-CMS 617664 — FEBRUARY 02, 2022
Subscribe to:
Posts (Atom)